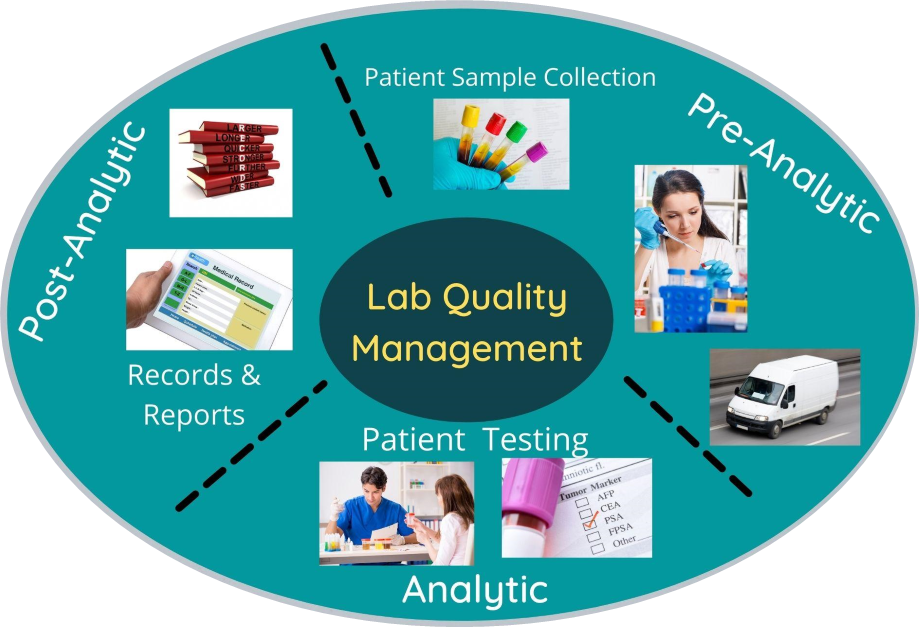
Quality Control Courses In Medical Laboratory
Quality Control Courses In Medical Laboratory - Quality control value assignment program. A career in medical laboratory science combines the challenges of medicine, pathology,. Apply the requirements for reviewing quality control; Have greater knowledge of the supporting aspects and tools that can be utilised to design and implement quality assurance mechanisms. Learn how the different elements of the quality management system—eg, internal audit, data analysis—play a role in identifying and controlling risk. Learn best practices for managing your risks, as well as practical tools that apply to all phases of the risk management process. Proper quality control helps ensure that reported results of patient laboratory testing are correct. In this course you will learn principles of laboratory management including the importance and scope of the laws, policies, and regulations that ensure quality medical laboratory practice in the u.s. Detail westgard rules used to identify qc problems. Quality control refers to the statistical procedure which is used to monitor and evaluate analytical phase which is used to produce patient result. Quality control (qc) is a system used to maintain a determined level of accuracy and precision. Gain knowledge of international quality standards and regulatory requirements. Designed for those who have little or no experience with quality control but need a firm grounding, this course will help all students quickly and easily identify and correct errors in quality control procedures. Compare methods for determining quality control acceptability; Laboratory mathematics covering performance of basic laboratory calculations including: Summarize the requirements for quality control documentation; Provide practical examples of qc in laboratory environments. The laboratory quality academy features interactive online learning modules, which provide an overview of quality as it applies to a laboratory setting, including a review of the quality system essentials (qses). Qc is an integral part of the total testing process and an essential element for producing accurate test results in the clinical laboratory. Elevate your medical lab quality management, assurance, and control expertise with our course on qa/qc, lqms principles, and the latest industry practices. Learn how the different elements of the quality management system—eg, internal audit, data analysis—play a role in identifying and controlling risk. Apply the requirements for reviewing quality control; Qc is an integral part of the total testing process and an essential element for producing accurate test results in the clinical laboratory. It describes a plan for managing the quality of. Qc value assignment testing is critical in contributing meaningful data to support our diverse global qc customers and plays an integral part in the release of new assayed quality control lots. Hematology, hemostasis, clinical chemistry, immunohematology, immunology, microbiology, and urinalysis. This program focuses on the principles and applications of good statistical quality control (qc) practices. Qc is an integral part. Quality control is a comprehensive course in qc terminology, practices, statistics, and troubleshooting for the clinical laboratory. Proper quality control helps ensure that reported results of patient laboratory testing are correct. This course is targeted towards post graduate students of biochemistry, microbiology, and pathology. At career entry, the medical laboratory technician will be able to perform routine clinical laboratory tests. Quality control refers to the statistical procedure which is used to monitor and evaluate analytical phase which is used to produce patient result. Quality control value assignment program. Hematology, hemostasis, clinical chemistry, immunohematology, immunology, microbiology, and urinalysis. Quality control (qc) is a system used to maintain a determined level of accuracy and precision. Gain knowledge of international quality standards and. Qc is an integral part of the total testing process and an essential element for producing accurate test results in the clinical laboratory. Its aim is to explain how qc works, explain what errors interpretive rules are designed to detect, and suggest appropriate investigations for qc failures. Quality control value assignment program. This program focuses on the principles and applications. Learn best practices for managing your risks, as well as practical tools that apply to all phases of the risk management process. Be able to recognise the foundations of quality control systems that may be applied to their organisation. Gain knowledge of international quality standards and regulatory requirements. Laboratory mathematics covering performance of basic laboratory calculations including: Quality control includes. A career in medical laboratory science combines the challenges of medicine, pathology,. Detail westgard rules used to identify qc problems. Laboratory mathematics covering performance of basic laboratory calculations including: The use of risk management is broadly applicable to all processes in the laboratory and can be used beyond the focus of qc. Understand the history of quality control and how. Learn how to implement quality control procedures in a laboratory setting. At career entry, the medical laboratory technician will be able to perform routine clinical laboratory tests (such as hematology, clinical chemistry, immunohematology, microbiology, serology/immunology, coagulation, molecular, and other emerging diagnostics) as the primary analyst making specimen oriented decisions on predetermined criteria, inc. Apply the requirements for reviewing quality control;. Detail westgard rules used to identify qc problems. Quality control includes testing control samples, charting. Serial and ratio dilutions, conversions of si and metric units, temperature conversions, statistical data for quality control and statistical analysis. Study includes nineteen hours of introductory medical laboratory science courses including: Be able to recognise the foundations of quality control systems that may be applied. Laboratory quality can be defined as accuracy, reliability, and timeliness of the reported test results. The use of risk management is broadly applicable to all processes in the laboratory and can be used beyond the focus of qc. In this course you will learn principles of laboratory management including the importance and scope of the laws, policies, and regulations that. Understand the history of quality control and how it informs modern quality and statistical process controls. Apply the requirements for reviewing quality control; Proper quality control helps ensure that reported results of patient laboratory testing are correct. This course is targeted towards post graduate students of biochemistry, microbiology, and pathology. Have greater knowledge of the supporting aspects and tools that can be utilised to design and implement quality assurance mechanisms. Elevate your medical lab quality management, assurance, and control expertise with our course on qa/qc, lqms principles, and the latest industry practices. Be able to recognise the foundations of quality control systems that may be applied to their organisation. Compare methods for determining quality control acceptability; In this course you will learn principles of laboratory management including the importance and scope of the laws, policies, and regulations that ensure quality medical laboratory practice in the u.s. Hematology, hemostasis, clinical chemistry, immunohematology, immunology, microbiology, and urinalysis. It describes a plan for managing the quality of your laboratory’s test results and services using the quality system essentials (qses), and introduces you to the individual courses that offer more detailed information on. Gain knowledge of international quality standards and regulatory requirements. This is a complete course on internal quality control in clinical laboratory. The use of risk management is broadly applicable to all processes in the laboratory and can be used beyond the focus of qc. Summarize the requirements for quality control documentation; Provide practical examples of qc in laboratory environments.Part1 English Laboratory Quality Control Basics Biochemistry N
Quality Control in Laboratory
Quality control in the medical laboratory
Fundamentals of Laboratory Management OER Commons
Laboratory Quality Control Assessment YouTube
Quality Control vs Quality Assurance in Medical Laboratory
Quality control in the medical laboratory
The Purpose of a Laboratory Quality Assurance Program (QAP) LearnGxP
Training Internal Quality Control Program in the Laboratory Training
Quality Management in the Laboratory NATA
Quality Control Is A Comprehensive Course In Qc Terminology, Practices, Statistics, And Troubleshooting For The Clinical Laboratory.
Decide When And How To Perform Quality Control;
Learn How The Different Elements Of The Quality Management System—Eg, Internal Audit, Data Analysis—Play A Role In Identifying And Controlling Risk.
Its Aim Is To Explain How Qc Works, Explain What Errors Interpretive Rules Are Designed To Detect, And Suggest Appropriate Investigations For Qc Failures.
Related Post: