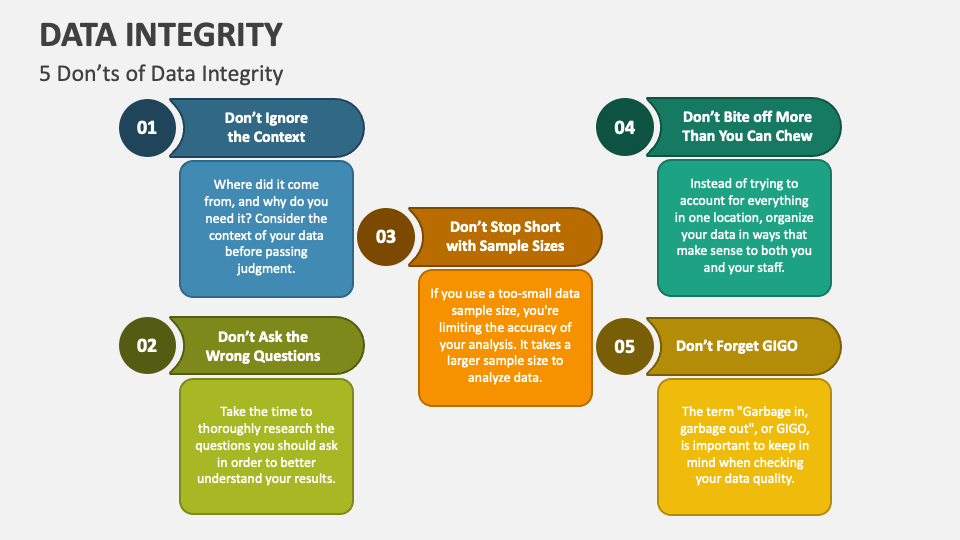
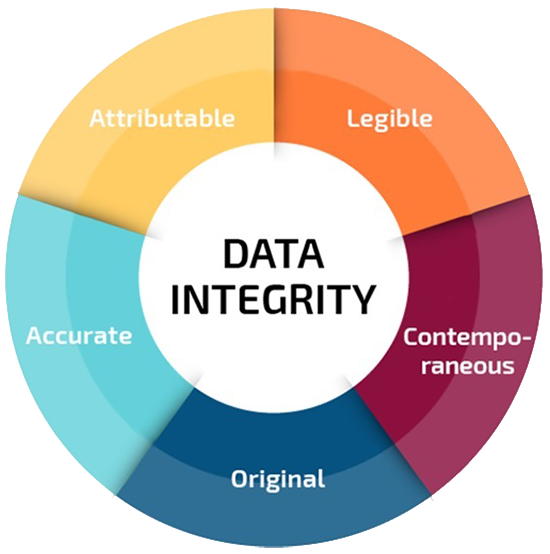
Data Integrity Course
Data Integrity Course - This course equips learners with the essential knowledge and skills to uphold data integrity in their everyday situations. Dr kylie hunter and prof anna lene seidler specialise in collaborative methodologies to improve the integrity and quality of research evidence via systematic. Learn data integrity, earn certificates with paid and free online courses from stanford, johns hopkins, university of virginia, mephi and other top universities around the world. Learn what is required for a data governance system from senior management. Data integrity, you'll learn to implement and maintain robust data integrity measures as part of your data security strategy. Understand the fda requirements for data integrity, mhra data integrity guidance and who guidance. A cgmp data integrity elearning course to help you comply with industry standards for ensuring accurate and reliable data during drug development, pharmaceutical manufacturing, and. This course will cover data integrity, electronic records and signatures, and the compliant operation of gxp computerized systems to provide the tools and techniques to implement. In the gmp certification programme “data integrity manager”, participants obtain a comprehensive knowledge of the basic principles of data integrity and data governance. In this course, we will explore the foundations of data integrity. Management and the quality unit need to have a deeper understanding of data integrity to give them the confidence to get involved and this module is designed to highlight the known. The importance and amount of data being generated to ensure product quality and patient safety continues to grow, and. Learn data integrity, earn certificates with paid and free online courses from stanford, johns hopkins, university of virginia, mephi and other top universities around the world. Why is data integrity so important? This course will cover data integrity, electronic records and signatures, and the compliant operation of gxp computerized systems to provide the tools and techniques to implement. This course will equip you with the necessary knowledge to. Data integrity is a critical issue in document governance and is increasingly becoming a focus for regulatory inspections globally. They’ll delve into the alcoa+ framework, a cornerstone for ensuring. Understand the fda requirements for data integrity, mhra data integrity guidance and who guidance. This course will cover data integrity, electronic records and signatures, and the compliant operation of gxp computerized systems to provide the tools and techniques to implement. Applying quality risk management to data integrity. Understand the fda requirements for data integrity, mhra data integrity guidance and who guidance. This course will cover data integrity, electronic records and signatures, and the compliant operation of gxp computerized systems to provide the tools and techniques to implement. In this course, we will explore the foundations of data integrity. This course. A cgmp data integrity elearning course to help you comply with industry standards for ensuring accurate and reliable data during drug development, pharmaceutical manufacturing, and. This course will equip you with the necessary knowledge to. In the gmp certification programme “data integrity manager”, participants obtain a comprehensive knowledge of the basic principles of data integrity and data governance. The importance. Data integrity is a critical issue in document governance and is increasingly becoming a focus for regulatory inspections globally. They’ll delve into the alcoa+ framework, a cornerstone for ensuring. This course will cover data integrity, electronic records and signatures, and the compliant operation of gxp computerized systems to provide the tools and techniques to implement. In the gmp certification programme. They’ll delve into the alcoa+ framework, a cornerstone for ensuring. This course will cover data integrity, electronic records and signatures, and the compliant operation of gxp computerized systems to provide the tools and techniques to implement. We first start with the importance of data integrity before learning the principles of alcoa++. This course equips learners with the essential knowledge and. We first start with the importance of data integrity before learning the principles of alcoa++. Understand the fda requirements for data integrity, mhra data integrity guidance and who guidance. Why is data integrity so important? In this course, data security champion: The importance and amount of data being generated to ensure product quality and patient safety continues to grow, and. Applying quality risk management to data integrity. The importance and amount of data being generated to ensure product quality and patient safety continues to grow, and. Data integrity is a critical issue in document governance and is increasingly becoming a focus for regulatory inspections globally. In this course, data security champion: Learn data integrity, earn certificates with paid and free. Applying quality risk management to data integrity. Subscribe to learningjoin 69m+ learnersstay updated with aiexpert instuctors Learn data integrity, earn certificates with paid and free online courses from stanford, johns hopkins, university of virginia, mephi and other top universities around the world. Why is data integrity so important? A cgmp data integrity elearning course to help you comply with industry. Understand the fda requirements for data integrity, mhra data integrity guidance and who guidance. Why is data integrity so important? We first start with the importance of data integrity before learning the principles of alcoa++. A cgmp data integrity elearning course to help you comply with industry standards for ensuring accurate and reliable data during drug development, pharmaceutical manufacturing, and.. In this course, we will explore the foundations of data integrity. Why is data integrity so important? Understand the fda requirements for data integrity, mhra data integrity guidance and who guidance. Learn data integrity, earn certificates with paid and free online courses from stanford, johns hopkins, university of virginia, mephi and other top universities around the world. Data integrity is. Management and the quality unit need to have a deeper understanding of data integrity to give them the confidence to get involved and this module is designed to highlight the known. This course will cover data integrity, electronic records and signatures, and the compliant operation of gxp computerized systems to provide the tools and techniques to implement. This training program. A cgmp data integrity elearning course to help you comply with industry standards for ensuring accurate and reliable data during drug development, pharmaceutical manufacturing, and. Subscribe to learningjoin 69m+ learnersstay updated with aiexpert instuctors Dr kylie hunter and prof anna lene seidler specialise in collaborative methodologies to improve the integrity and quality of research evidence via systematic. This course will equip you with the necessary knowledge to. This course will cover data integrity, electronic records and signatures, and the compliant operation of gxp computerized systems to provide the tools and techniques to implement. This course will cover data integrity, electronic records and signatures, and the compliant operation of gxp computerized systems to provide the tools and techniques to implement. This training program has been developed to train employees with intermediate knowledge of data integrity (alcoa). The importance and amount of data being generated to ensure product quality and patient safety continues to grow, and. In this course, we will explore the foundations of data integrity. Learn data integrity, earn certificates with paid and free online courses from stanford, johns hopkins, university of virginia, mephi and other top universities around the world. We first start with the importance of data integrity before learning the principles of alcoa++. In the second half of the course,. Data integrity is a critical issue in document governance and is increasingly becoming a focus for regulatory inspections globally. They’ll delve into the alcoa+ framework, a cornerstone for ensuring. Applying quality risk management to data integrity. In the gmp certification programme “data integrity manager”, participants obtain a comprehensive knowledge of the basic principles of data integrity and data governance.How to Ensure Data Integrity Simple Strategies for Reliable Software
Data Integrity PowerPoint and Google Slides Template PPT Slides
What is data integrity? (The Definition, Types and Risks)
The Fundamentals of Data Integrity LearnGxP Accredited Online Life
Data Integrity Training by Dr. A. Amsavel
Data Integrity PowerPoint and Google Slides Template PPT Slides
DATA INTEGRITY Foundation of Compliance; Critical Source of FDA
Get Your Data Integrity Basics Down for Success FIVE Validation
The Fundamentals of Data Integrity LearnGxP Accredited Online Life
Data Integrity Overview What It Is And How To Preserv vrogue.co
This Course Equips Learners With The Essential Knowledge And Skills To Uphold Data Integrity In Their Everyday Situations.
Understand The Fda Requirements For Data Integrity, Mhra Data Integrity Guidance And Who Guidance.
Learn What Is Required For A Data Governance System From Senior Management.
Management And The Quality Unit Need To Have A Deeper Understanding Of Data Integrity To Give Them The Confidence To Get Involved And This Module Is Designed To Highlight The Known.
Related Post: