Citi Refresher Course
Citi Refresher Course - All uic investigators and key research personnel* are required to complete the initial investigator training requirements in human subject protections, a citi human subjects protections (hsp). It covers historical and current information. The refresher courses are shorter than the original training course but will contain any new or revised information a researcher would need. You are required to renew your citi training every three years by taking the relevant refresher course(s). Peoria irb requires its irb chairs, members, alternates and researchers to complete the citi refresher courses every three years following the initial education program. Then click the next button at the. Gcp ich refresher offers retraining on gcp for clinical trials with investigational drugs and biologics (ich focus). Loyola university chicago uses the online training course called the collaborative irb training initiative (citi) course to provide convenient web based training programs to the research. View details at citi program. Human research curriculum (satisfies irb certification requirements, not rcr) refresher course (the basic course must have been completed previously). You are required to renew your citi training every three years by taking the relevant refresher course(s). Stanford provides access to required training through an interactive online tutorial, the citi. Loyola university chicago uses the online training course called the collaborative irb training initiative (citi) course to provide convenient web based training programs to the research. Citi program provides training courses for colleges and universities, healthcare institutions, technology and research organizations, and governmental agencies. After you log into the citi site, the. It covers historical and current information. Citi rcr refresher stage (complete once every 2 years) will i receive a reminder email to let me know my rcr training will soon expire? It covers historical and current information on regulatory. Then click the next button at the. Listing of rcr refresher continuing education certified modules and requirements to apply for ce credits for this course It covers historical and current information. Peoria irb requires its irb chairs, members, alternates and researchers to complete the citi refresher courses every three years following the initial education program. It covers historical and current information on regulatory. Stanford provides access to required training through an interactive online tutorial, the citi. The refresher courses are shorter than the original training. Citi program provides training courses for colleges and universities, healthcare institutions, technology and research organizations, and governmental agencies. How do i complete the refresher course? Peoria irb requires its irb chairs, members, alternates and researchers to complete the citi refresher courses every three years following the initial education program. All uic investigators and key research personnel* are required to complete. It covers historical and current information on regulatory. It covers historical and current information. After you have completed a basic or refresher course, citi will send the vanderbilt human research protection program (vhrpp) an email within. If you remain engaged in human subject research, the citi training refresher course must be completed every three (3) years. You are required to. The refresher courses are shorter than the original training course but will contain any new or revised information a researcher would need. Human research curriculum (satisfies irb certification requirements, not rcr) refresher course (the basic course must have been completed previously). Click on the link to complete the course. After you log into the citi site, the. Required tutorial on. Stanford provides access to required training through an interactive online tutorial, the citi. You are required to renew your citi training every three years by taking the relevant refresher course(s). Required tutorial on human subject research protection and good clinical practice. Click on the link to complete the course. If you remain engaged in human subject research, the citi training. Human research curriculum (satisfies irb certification requirements, not rcr) refresher course (the basic course must have been completed previously). It covers historical and current information. It covers historical and current information. After you log into the citi site, the. It covers historical and current information on regulatory. Click on the link to complete the course. Loyola university chicago uses the online training course called the collaborative irb training initiative (citi) course to provide convenient web based training programs to the research. Learners will gain a solid understanding of how to design quantitative, qualitative, and mixed methods studies, collect and analyze data, and draw meaningful conclusions across various.. Human research curriculum (satisfies irb certification requirements, not rcr) refresher course (the basic course must have been completed previously). Citi program provides training courses for colleges and universities, healthcare institutions, technology and research organizations, and governmental agencies. Loyola university chicago uses the online training course called the collaborative irb training initiative (citi) course to provide convenient web based training programs. Peoria irb requires its irb chairs, members, alternates and researchers to complete the citi refresher courses every three years following the initial education program. Online rcr course through citi; Required tutorial on human subject research protection and good clinical practice. The refresher courses are shorter than the original training course but will contain any new or revised information a researcher. The refresher courses are shorter than the original training course but will contain any new or revised information a researcher would need. All uic investigators and key research personnel* are required to complete the initial investigator training requirements in human subject protections, a citi human subjects protections (hsp). After you log into the citi site, the. Citi rcr refresher stage. Stanford provides access to required training through an interactive online tutorial, the citi. The refresher courses are shorter than the original training course but will contain any new or revised information a researcher would need. Then click the next button at the. Required tutorial on human subject research protection and good clinical practice. Citi program provides training courses for colleges and universities, healthcare institutions, technology and research organizations, and governmental agencies. Learners will gain a solid understanding of how to design quantitative, qualitative, and mixed methods studies, collect and analyze data, and draw meaningful conclusions across various. After you have completed a basic or refresher course, citi will send the vanderbilt human research protection program (vhrpp) an email within. After you log into the citi site, the. Click on the link to complete the course. It covers historical and current information. Listing of rcr refresher continuing education certified modules and requirements to apply for ce credits for this course Loyola university chicago uses the online training course called the collaborative irb training initiative (citi) course to provide convenient web based training programs to the research. If you remain engaged in human subject research, the citi training refresher course must be completed every three (3) years. How do i complete the refresher course? Online rcr course through citi; Human research curriculum (satisfies irb certification requirements, not rcr) refresher course (the basic course must have been completed previously).Training & Certification Institutional Review Board Teachers
Citi Training GCP and Refresher Exam Questions with Correct Answers
CITI Social and Behavioral Research Basic.Refresher Course PDF
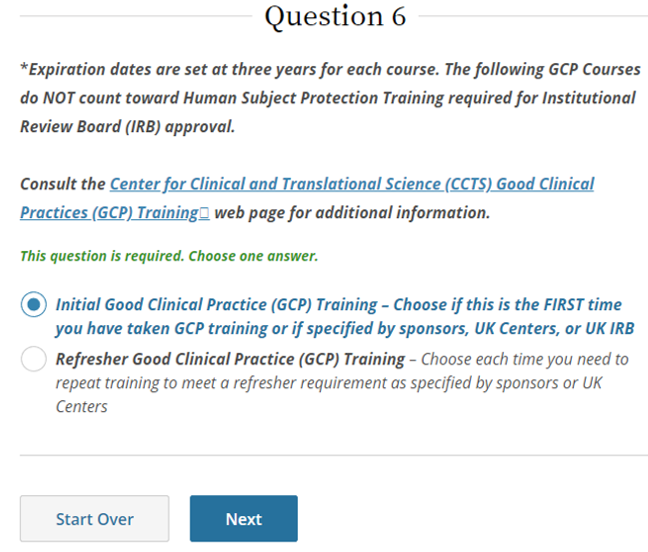
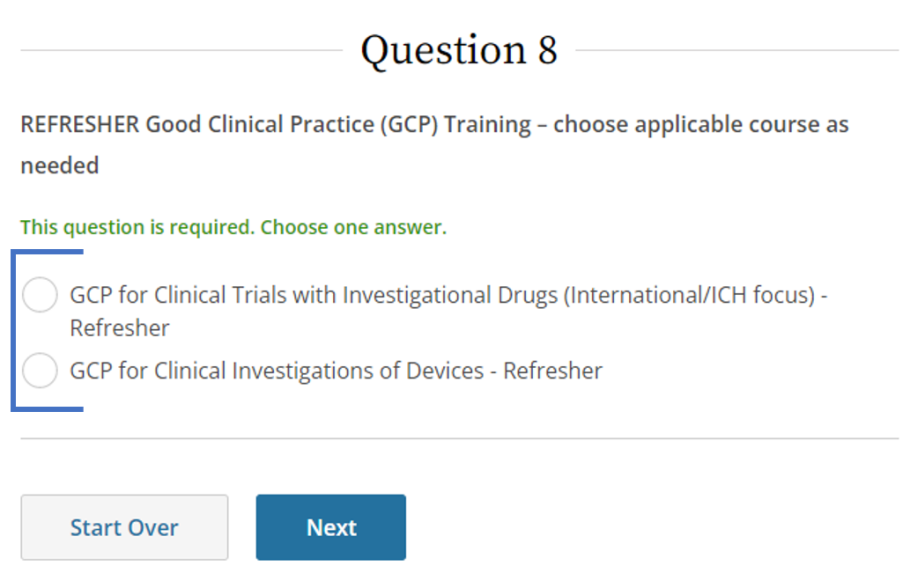
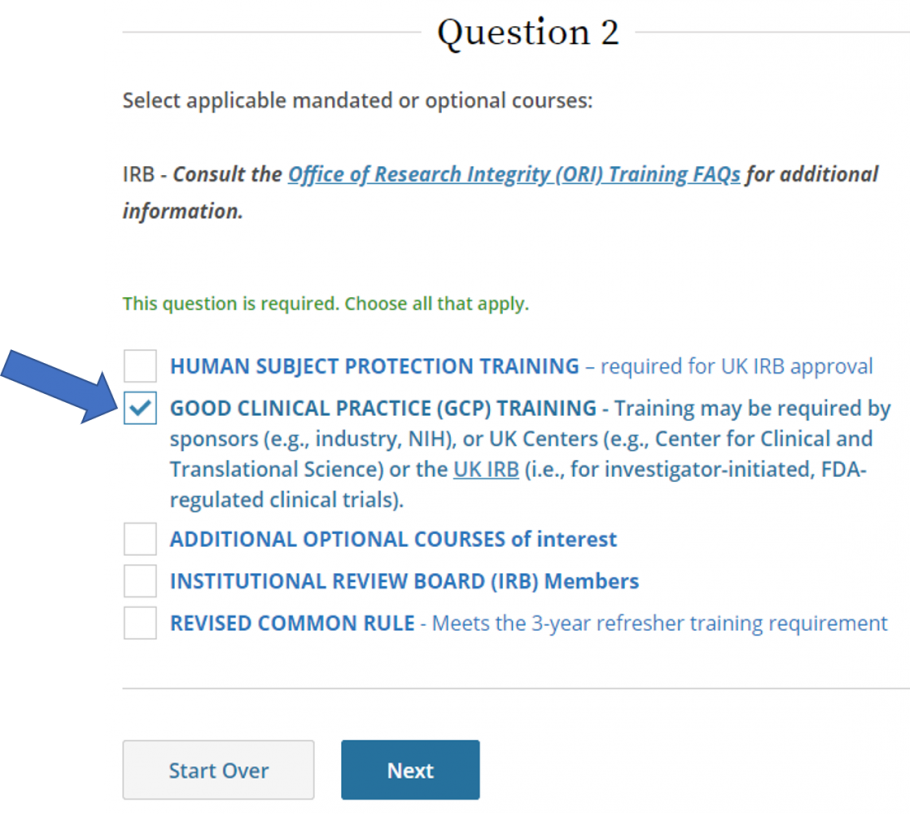
SponsorInvestigator (GCP) CITI Training FAQs University of Kentucky
CITI Social Behavioral Science Basic/Refresher Training Access YouTube
Reading and Understanding a CITI Program Completion Report and
SponsorInvestigator (GCP) CITI Training FAQs University of Kentucky
Guide to GCP Mutual Recognition Completion Documentation
SponsorInvestigator (GCP) CITI Training FAQs University of Kentucky
Required Ethics Training Committee on the Use of Human Subjects
Peoria Irb Requires Its Irb Chairs, Members, Alternates And Researchers To Complete The Citi Refresher Courses Every Three Years Following The Initial Education Program.
View Details At Citi Program.
All Uic Investigators And Key Research Personnel* Are Required To Complete The Initial Investigator Training Requirements In Human Subject Protections, A Citi Human Subjects Protections (Hsp).
It Covers Historical And Current Information On Regulatory.
Related Post: